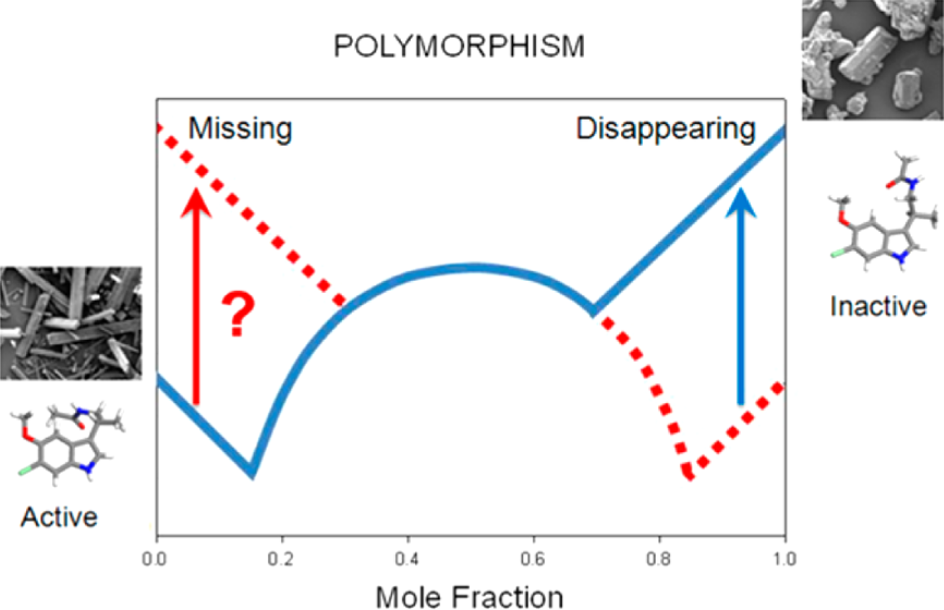
Consultation is available in numerous areas of pharmaceutical sciences. These services may range from oversight of the screening for the optimal form for your API or pharmaceutical product by searching its “solid-form landscape” for physical forms by screening for salts, co-crystals, and amorphous forms. Services also include oversight of the collection and interpretation of characterization data and chemical and physical stability (polymorphic, hydrate, or disproportionation/dissociation) data leading to the optimal physical form selection for your product’s presentation.
Greg Stephenson has a knack for solution of challenging problems of physical form, having solved numerous serious problems in drug development involving control of physical forms that impact dissolution rate, control of crystal morphology that impact flow characteristics and the processing/tableting of materials. We also offer services with respect to evaluation of patents, the structuring of claims for crystal forms in patents and their defense or questions of validity through litigation.
Our consultation services can act directly as an integrated team member of discovery chemists, pharmaceutical scientists, process chemists, analytical chemists and formulation scientists and regulatory teams. Alternatively, we serve independently to solve your problems of solid-state pharmaceutics by accessing external network of CROs to obtain data to solve your pharmaceutical challenges.